An atom of matter is like a tiny solar system. It has a central sun called a nucleus round which move the planets of the system, the electrons.
The diameter of the nucleus is only about ten-thousandth of that of an atom, yet the nucleus is itself a complete body built up from particles called protons and neutrons.
When matter undergoes a chemical reaction such as burning, for example the planetary elcectrons of its atoms are rearanged and as a consequence energy is released. This energy usually appears as heat, as the heat of a coal fire, or a light in a gas flame. In these reactions the neclei of the atoms taking part are undisturbed.
In nuclear reactions, however, the nucleus itself is disturbed or even broken up and very much more energy is released, But nuclear reactions, unlike chemical reactions cannot generally be made to spread from one atom to the next; each atom has to be treated individually. Fortunately there is one exception - the reactor called nuclear fission, which is the cornerstone of nuclear power.
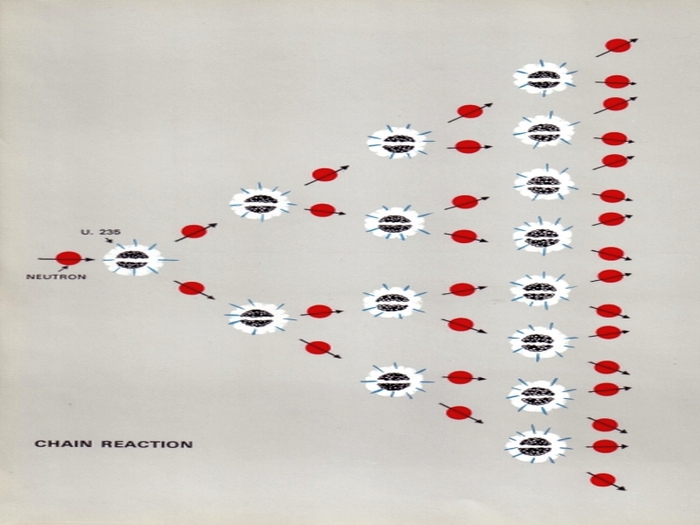
Fission is produced when a nucleus of certain heavy elements is struck by a neutron. The nucleus absorbs the neutron, its equilibrium is disturbed and it is split into two much more or less equal parts. In thsi splitting there is a release of energy and also of more than one fresh neutron (about 2.5 on avearge). These new neutrons then cause further fissions in neighbouring atoms and these in turn release more neutrons to cause yet another generation of fissions and so on.
In this way there is produced a self-sustaining chain reaction.
The energy released in fission is imparted to the two fragments into which the nucleus is split, causing them to move apart with great speed. This motion creates heat and it is this geat which is removed and converted - by means of steam - to mechanical energy and then into electricity.
Uranium, a metal heavier than head is the material used, It is only naturally occurring material which will sustain a nuclear chain reaction.
The diameter of the nucleus is only about ten-thousandth of that of an atom, yet the nucleus is itself a complete body built up from particles called protons and neutrons.
When matter undergoes a chemical reaction such as burning, for example the planetary elcectrons of its atoms are rearanged and as a consequence energy is released. This energy usually appears as heat, as the heat of a coal fire, or a light in a gas flame. In these reactions the neclei of the atoms taking part are undisturbed.
In nuclear reactions, however, the nucleus itself is disturbed or even broken up and very much more energy is released, But nuclear reactions, unlike chemical reactions cannot generally be made to spread from one atom to the next; each atom has to be treated individually. Fortunately there is one exception - the reactor called nuclear fission, which is the cornerstone of nuclear power.
Fission is produced when a nucleus of certain heavy elements is struck by a neutron. The nucleus absorbs the neutron, its equilibrium is disturbed and it is split into two much more or less equal parts. In thsi splitting there is a release of energy and also of more than one fresh neutron (about 2.5 on avearge). These new neutrons then cause further fissions in neighbouring atoms and these in turn release more neutrons to cause yet another generation of fissions and so on.
In this way there is produced a self-sustaining chain reaction.
The energy released in fission is imparted to the two fragments into which the nucleus is split, causing them to move apart with great speed. This motion creates heat and it is this geat which is removed and converted - by means of steam - to mechanical energy and then into electricity.
Uranium, a metal heavier than head is the material used, It is only naturally occurring material which will sustain a nuclear chain reaction.